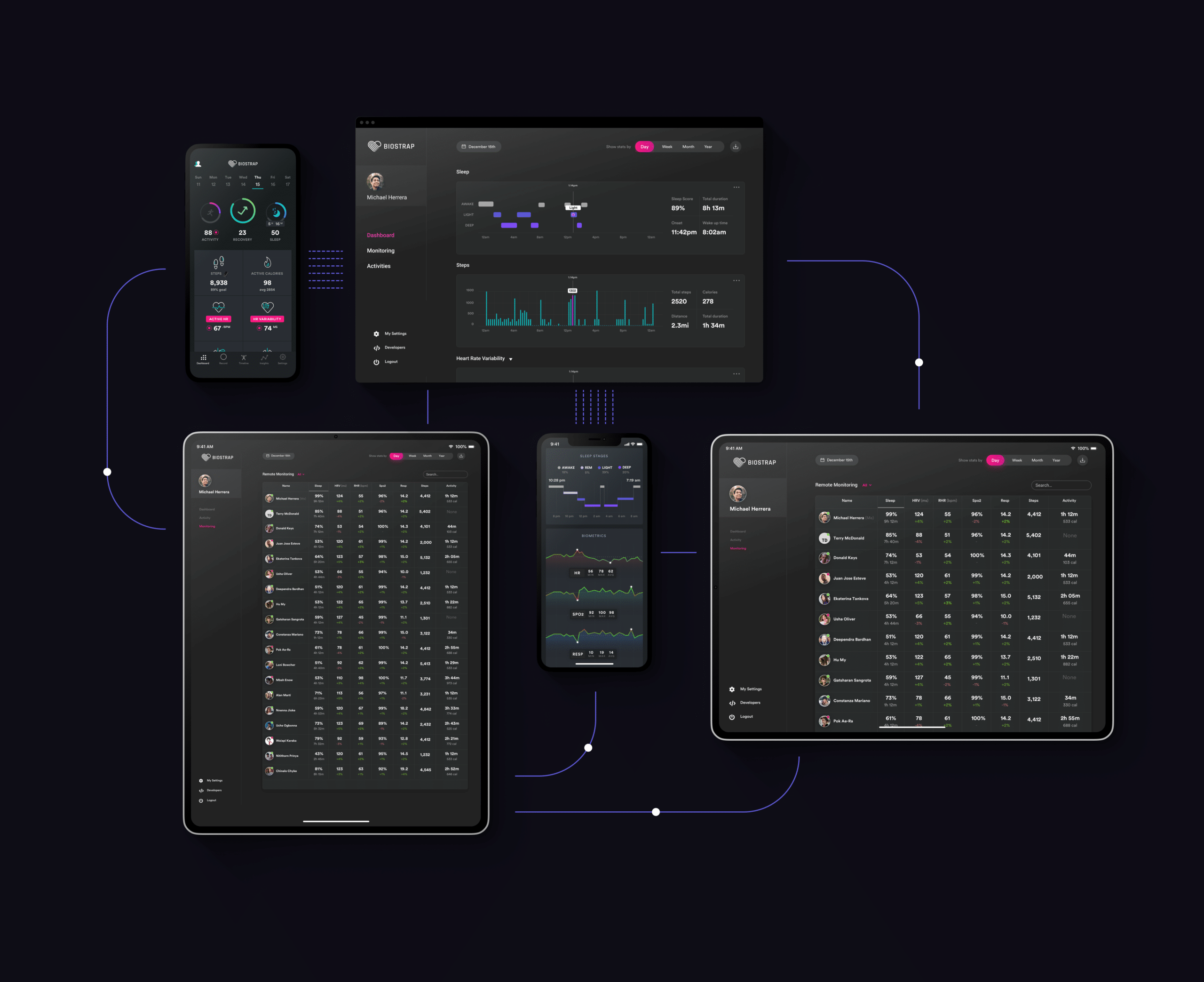
Validation & Studies
Data integrity is critical when relied upon for remote monitoring, risk stratification, and decision-making. Through partnering with universities and other research organizations, Biostrap’s biometric measurements have been validated against gold standard diagnostic equipment in 14 publications and 22 clinical studies.